Chemistry in biotic interactions: The many facets of cuticular hydrocarbons
Many insects coat their body surfaces with cuticular hydrocarbons (alkanes, alkenes, etc. with various chain lengths), which act as desiccation resistance and communication tool. Florian Menzel from the Johannes Gutenberg University Mainz investigates such cuticular hydrocarbons in ants and recently revealed that CHC composition and profiles differ across species but also body parts of the same individual. Here, Florian highlights the main points of CHC research.
A View by Florian Menzel
All pictures are © Florian Menzel.

What has always fascinated me is the question of which, and how, different organisms interact. Especially in the tropics, it is just amazing how many curious forms of interaction you can find by observing nature. For my PhD, I studied two species of parabiotic ants in the tropical rainforest of Borneo, the larger Camponotus rufifemur and the smaller Crematogaster modiglianii, which – unusual enough – do not fight against each other. Quite the contrary – they live together in the same nest. They even feed each other. And if you keep them separate for a day and re-unite them, Crematogaster workers get very excited and crawl upon the Camponotus workers’ backs [1].

The two parabiotic species, Camponotus rufifemur and Crematogaster modiglianii, foraging together. 
So, the two ant species live together in harmony. But that is just the start. They live together with an epiphyte: Poikilospermum cordifolium. In fact, when you search for these ants, you can find them much faster if you look for the plant instead of for the ants. The ants are attracted to the seeds and carry them into the nest where they germinate. That is why such nests are also called ‘ant gardens’ – gardens planted by the ants, an ability perfectioned by their neotropical colleagues, Camponotus femoratus and Crematogaster levior. These guys (they are my current research subjects) build aesthetic ant gardens, with Philodendron, Aechmea bromeliads, and really anything you can think of if you like aesthetic plant arrangements.

A commensalistic Colobopsis species at a bait visited by Crematogaster modiglianii (Borneo). 
Crematogaster modiglianii carrying a seed of the epiphyte Poikilospermum cordifolium. 
Parabiotic nest in Borneo, with the epiphyte P. cordifolium. 
Parabiotic nest in South America (French Guiana), with the epiphytes Aechmea mertensii and Philodendron sp.
The ants share food trails too and often forage next to each other in (relative) peace. Around many parabiotic nests, another ant (genus Colobopsis) will come along and forage beside the two parabiotic ants, although they usually attack it [2]. Also, the two parabiotic ants tend trophobionts together. At one site we found red thingies, which turned out to be some Rhynchota integuments full of offspring (I could not identify them further). The ants even build carton shelters for these critters. How strange is that!? It always stuns me to see such specialised associations – or entire networks of interspecific interactions. How did this evolve?
Even when we just focus on the two main players, Camponotus and Crematogaster, there is plenty to discover. Trail-sharing of Camponotus and Crematogaster species is common worldwide, including Southern Europe [3]. Besides the Bornean parabiosis, there is a convergent one in tropical South America [4], and a quite similar (but less studied) association in Papua New Guinea. Why always these two genera? What makes them so special? [5]
It was rather by chance that I encountered cuticular hydrocarbons when trying to answer these questions. We first tried to find out whether parabiotic ants have similar CHCs – chemical mimicry is common in many parasite-host associations, so why not here? But at first, there were no hydrocarbons in the extracts. In extracts of non-parabiotic species yes, but not in those of parabiotic ones. We even started writing a grant proposal that should deal with the question of why parabiotic ants have no CHCs. Only after that, we realised that we need special analytical methods to detect parabiotic CHCs – because they are longer than those of other species, so you will not detect them with ‘conventional’ GC-MS analyses [6].
So why these long-chain CHCs? What started with a simple question became one of my research foci – the causes and consequences of CHC variation within and across species (see [7] for an overview of the current research). Why is that so exciting? Because CHCs are a multifunctional tool – they make the insect body waterproof, and they serve as a communication signal, carrying multiple pieces of information at the same time. They might also serve as a lubricant of limb joints and probably protect against fungal infection. Finally, hydrocarbon droplets are left in the environment as a footprint, possibly to enhance foot adhesion. How does an insect manage to optimize all these different functions? Many functions mean many different requirements to ensure functionality. So which selection pressures shape hydrocarbon profiles? And which traits are actually selected for?
We found that CHC composition differs between species from wet (or alpine) and dry habitats [8]. Apparently, alkenes are better when it is wet or cool. But why? Chemically, they are not very different (besides being less inert). What differs are the physical properties – how the molecules aggregate, and hence their phase behaviour. Although this has not received much attention from chemical ecologists so far, hydrocarbons are not all solid – they have very different phase behaviours. As we found out recently, some CHCs already melt at -45°C, and the last CHCs stay solid until about +40°C [9]. This means that a CHC layer always contains solid and liquid parts, like melted chocolate with some solid chunks in it. When it gets warmer, more solid chunks will melt, and the liquid parts will become less viscous. This makes them easier to perceive (hence facilitating communication), but it’s bad for waterproofing. So the ants need to adjust their CHC composition when they want to maintain a waterproof CHC layer. Currently, we are trying to find out how CHCs change during acclimation, and how this affects their information content, but also their material properties.

The parabiotic Camponotus rufifemur from the jungles of Borneo with one of its trophobionts. 
The same red ‘thingies’ opened under the microscope.
Recently, we looked into differences in CHC profiles between different body parts of an ant. What seemed to be complete chaos of relative differences makes perfect sense when you sort the CHCs after biophysical criteria – some body parts contain rather solid (or viscous) CHCs, some more the liquid (and less viscous) ones. The distinction between early-melting and late-melting CHCs actually can help us to understand how different information is maintained in the same channel – e.g. how nestmate recognition cues can be exchanged among colony members, while the queen signal – or task-group signals – are exchanged to a much lower degree. Hence, biophysical differences between CHCs allow the maintenance of multiple information channels in the same CHC profile.
It is amazing how CHCs are involved in so many biotic interactions and abiotic selection pressures. Foraging ants respond to CHCs left on the soil as footprints and change their movement patterns accordingly [10] – a topic my PhD student Vanessa Menges is studying. Chemical communication plays a role in interactions between ants and their trophobionts, between ants and myrmecophilic insects (ant guests), or between slave-making species and their hosts. CHCs even matter in predator-prey interactions – and this is driven by physics again: Some spiders, the so-called ‘cribellate’ spiders, do not use glue in their threads. Instead, their threads possess a nanostructure that makes CHCs adhere to them, such that a prey insect is caught in the threads because its CHCs act as glue, as my colleague Anna-Christin Joel found out recently [11]. But – the effect depends on the CHC composition, because only the less viscous CHCs glue the insect to the thread.CHCs are just one of the many traits that determine how living organisms interact, and how they manage to survive in their environment. The enormous biodiversity out there, especially in the tropics, and all the different ways how organisms cope with the ‘everyday challenges’ of their lives, is fascinating and awe-inspiring. In times of climate change and unprecedented species loss, our challenge is now to save as many species and habitats as we can and manage ecosystems in a sustainable way.

Deinopis, a spider from French Guiana’s rainforests. The near-quadratic capture net it holds beneath its body consists of cribellate threads, whose nanostructure cause the blueish colour. 
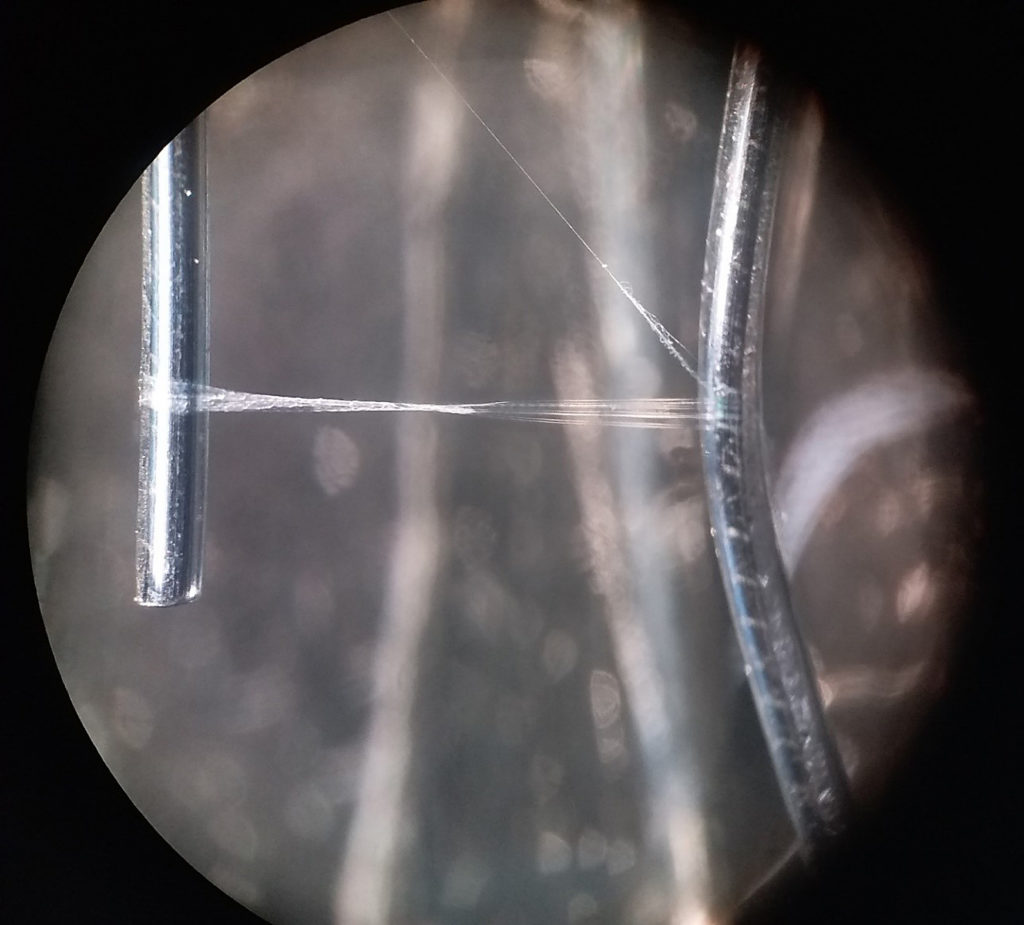
A cribellate spider thread (of Uloborus plumipes) spanned between two wires. The left half of the thread contains hydrocarbons that migrated into it – so this is one of the rare opportunities to actually see cuticular hydrocarbons!
References
[1] Menzel F, Blüthgen N (2010): Parabiotic associations between tropical ants: equal partner-ship or parasitic exploitation? Journal of Animal Ecology 79: 71-81
[2] Menzel F, Pokorny T, Blüthgen N, Schmitt T (2010): Trail-sharing among tropical ants: interspecific use of trail pheromones? Ecological Entomology 35: 495-503
[3] Menzel F, Woywod M, Blüthgen N, Schmitt T (2010): Behavioural and chemical mechanisms behind a Mediterranean ant-ant association. Ecological Entomology 35: 711-720
[4] Hartke J*, Sprenger PP*, Sahm J, Winterberg H, Orivel J, Baur H, Beuerle T, Schmitt T, Feldmeyer B*, Menzel F* (2019): Cuticular hydrocarbons as potential mediators of cryptic species divergence in a mutualistic ant association. Ecology and Evolution 9: 9160-9176 (*shared first/last authors)
[5] Menzel F, Orivel J, Kaltenpoth M, Schmitt T (2014): What makes you a potential partner? Insights from convergently evolved ant-ant symbioses. Chemoecology 24: 105-119
[6] Menzel F, Schmitt T (2012): Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66-3: 896-904
[7] Sprenger PP, Menzel F (2020): Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: how and why they differ among individuals, colonies, and species. Myrmecological News 30: 1-26
[8] Menzel F, Blaimer BB, Schmitt T (2017): How do cuticular hydrocarbons evolve? Physiological and climatic constraints on a complex functional trait in insects. Proceedings of the Royal Society B 284: 20161727
[9] Menzel F, Morsbach S, Martens JH, Räder P, Hadjadje S, Poizat M, Abou B (2019): Communication vs. waterproofing: the physics of insect cuticular hydrocarbons. Journal of Experimental Biology 222: jeb210807
[10] Wüst M, Menzel F (2017). I smell where you walked – how chemical cues influence move-ment decisions in ants. Oikos 126: 149-160
[11] Bott RA, Baumgartner W, Bräunig P, Menzel F, Joel A-C (2017): Adhesion enhancement of structural capture threads by epicuticular waxes of the insect prey sheds new light on spider web evolution. Proceedings of the Royal Society B 284: 20170363













Recent Comments