All Nurture, no Nature: Division of labor at r = 1
In the recently published paper “Stress and early experience underlie dominance status and division of labour in a clonal insect”, Abel Bernadou, Lukas Schrader, Julia Pable, Elisabeth Hoffacker, Karen Meusemann, and Jürgen Heinze revealed that differences in rearing conditions influence the division of labor in the clonal ant Platythyrea punctata. They also unveiled that division of labor was based on changes in gene regulation, which might also influence behavioral variation among genetically identical individuals. In this Review, Chris R. Smith highlights their main points.
A Review compiled by Chris R. Smith 
So much of our mechanistic knowledge in biology is derived from only a few species – these are model organisms like the mouse (Mus musculus), the fruit fly (Drosophila melanogaster), the roundworm (Caenorhabditis elegans), and E. coli bacteria, among others. But the obvious downside to working on any of these models is that they lack the amazing diversity of life on Earth – Darwin’s “endless forms most beautiful” (Darwin 1909). The benefits, though, are clear – for model organisms, so many tools are available, and genetic effects can be excluded by using completely inbred or clonal lines. Nonetheless they persisted: social insect biologists proclaim with clarity, conviction, and certainty why they don’t work on those few established models … of course, because they aren’t eusocial.
The rise of the clones – The last decades of social insect research saw an increase in the number of papers touting the benefits of genetic diversity within colonies (reviewed by Smith & al. 2008, Schwander & al. 2010). The costs of diversity within insect societies have always been clear and are ‘celebrated’ in Hamilton’s equation as the coefficient of relatedness (Hamilton 1964, Hughes & al. 2008). Nonetheless, diverse societies often outcompete their less diverse rivals through a myriad of benefits, from division of labor to immunity (Page & al. 1995, Liersch & Schmid-Hempel 1998, Hughes & Boomsma 2004, Mattila & Seeley 2007, Oldroyd & Fewell 2007, Hughes & Boomsma 2008). Despite the benefits of diversity, colonies of some species of ants are composed entirely of clones. And now we are entering a decade where research on clones is giving us some of the most fundamental insights into the way societies function (Hartmann & Heinze 2003, Bernadou & al. 2015, Trible & al. 2017, Bernadou & al. 2018, Chandra & al. 2018, Kronauer & Libbrecht 2018, Ulrich & al. 2018).

Workers, pupae and larvae of the thelytokous ant Platythyrea punctata. Workers change their cuticle colour with age from a yellowish to blackish coloration (worker body length approx. 8 mm). (© Abel Bernadou)
Division of labor among individuals is as fundamental to the operation of a society of clones as it is to a society where relatedness is low and genetic diversity contributes to variation in morphology, physiology, and behavior. And that clones have a division of labor turns out to be as fundamental to social evolution theory as the role of relatedness/diversity. That is, division of labor can be produced entirely via the regulation of shared processes. Furthermore, it is the environment, whether abiotic, social, or the interaction of the two, that regulates these processes. A recent paper by Abel Bernadou and co-authors in Proceedings of the Royal Society B used the clonal social organization of Platythyrea punctata colonies to reveal how environmental mechanisms contribute to division of labor (Bernadou & al. 2018).
In their study, Bernadou et al. used a division-of-labor assay whereby two age-matched clones are paired with each other and over time divide their labor, with one becoming behaviorally dominant and reproductive and the other tending to be more specialized at leaving the nest (foraging). The authors also modified the environments of the partners in further experiments to assay how specific environmental factors, like temperature, nutrition, stress, injury, and social environment (rearing with young or old workers) affected division of labor. This basic assay was complemented with head transcriptomics (RNAseq with de novo transcriptome assembly).
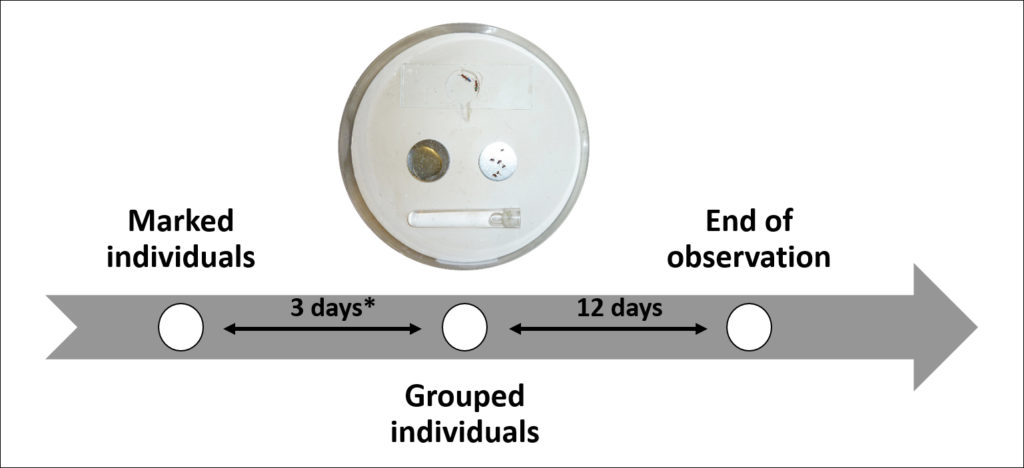
Experimental workflow used in the experiment. (© Abel Bernadou)
The environment experienced before the assay did indeed affect division of labor, mostly in predictable ways. For example, stressed or injured workers were more likely to be submissive and non-reproductive. Nutrition gave slightly surprising results, where the well-fed individuals did have more active ovaries than their hungrier counterparts but appeared to be behaviorally submissive (this observation could very well be an artifact of begging being scored as dominance behavior). Worker size only seemed to interact with dominance for individuals reared at different temperatures, with those reared at cooler temperatures being both larger and more dominant. Lastly, the social environment (previously being with young or older workers) did not have an observable effect on dominance.
The gene expression data from dominant and submissive individuals in clone pairs yielded unsurprising results, which nevertheless help build a beautiful picture of the role of genes and individuals in building a superorganism. In brief, some of the usual suspects (metabolic genes, hormone-responsive genes, and insulin signaling genes, among others) were differentially expressed between dominants and submissives. Nearly 40% of differentially expressed genes did not fit neatly into a clear functional category, and some of these did not have clear orthology to known genes in other organisms. The beauty in this result is that many of the ‘usual suspects’ have known functions in development or other contexts of caste difference and division of labor, not just behavioral variation. That is to say that there seems to be a core set of genes that govern responsiveness to the environment, and that these do tend to be typical regulators of division of labor. Phrased another way, there are a finite number of mechanisms a cell has to respond to the environment and so it is unsurprising that these environmentally responsive genes keep coming up in screens of division of labor. As individuals are made up of cells, and superorganisms made up of individuals, organizational phenomena in individuals and superorganisms are likely to be traced back to the same genes that provide cells with plastic responsiveness.

Colony of the clonal ant Platythyrea punctata in a nest in soil. (© Benjamin Barth)
This paper makes me think about the modularity of life across the ‘major transitions’ in evolution (i.e., from uni- to multi-cellularity, from asexuality to sexuality, from solitary to social, etc.) (Smith & Szathmary 1997). If you can get division of labor among clones by modifying their individual environments, and you can get division of labor among cells by doing the same thing (i.e., our own development is a stunning example of this), then it seems more clear that each major transition is fundamentally accompanied by changes in environment and/or changes in responsiveness to the environment.
In summary, this well-written and easily digestible paper by Bernadou et al. whets my appetite for more about what we can learn from social clones. After all, clonal superorganisms are much more prevalent in science fiction than societies composed of highly diverse individuals – and they do say that science fiction often predicts the future.

Typical habitat for Platythyrea punctata in Puerto Rico. (© Jürgen Heinze).
References
Bernadou, A., Busch, J. & Heinze, J. 2015: Diversity in identity: behavioral flexibility, dominance, and age polyethism in a clonal ant. – Behavioral Ecology and Sociobiology 69: 1365-1375.
Bernadou, A., Schrader, L., Pable, J., Hoffacker, E., Meusemann, K. & Heinze, J. 2018: Stress and early experience underlie dominance status and division of labour in a clonal insect. – Proceedings of the Royal Society of London B: Biological Sciences 285: 20181468.
Chandra, V., Fetter-Pruneda, I., Oxley, P.R., Ritger, A.L., McKenzie, S.K., Libbrecht, R. & Kronauer, D.J.C. 2018: Social regulation of insulin signaling and the evolution of eusociality in ants. – Science 361: 398-402.
Darwin, C. 1909: The Origin of Species. – P. F. Collier & Son, 562 pp.
Hamilton, W.D. 1964: The genetical evolution of social behaviour. I. – Journal of Theoretical Biology 7: 1-16.
Hartmann, A. & Heinze, J. 2003: Lay eggs, live longer: Division of labor and life span in a clonal ant species. – Evolution 57: 2424-2429.
Hughes, W.O.H. & Boomsma, J.J. 2004: Genetic diversity and disease resistance in leaf-cutting ant societies. – Evolution; International Journal of Organic Evolution 58: 1251-1260.
Hughes, W.O.H. & Boomsma, J.J. 2008: Genetic royal cheats in leaf-cutting ant societies. – Proceedings of the National Academy of Sciences 105: 5150-5153.
Hughes, W.O.H., Oldroyd, B.P., Beekman, M. & Ratnieks, F.L.W. 2008: Ancestral monogamy shows kin selection is key to the evolution of eusociality. – Science 320: 1213-1216.
Kronauer, D.J. & Libbrecht, R. 2018: Back to the roots: the importance of using simple insect societies to understand the molecular basis of complex social life. – Current Opinion in Insect Science 28: 33-39.
Liersch, S. & Schmid-Hempel, P. 1998: Genetic variation within social insect colonies reduces parasite load. – Proceedings of the Royal Society of London B: Biological Sciences 265: 221-225.
Mattila, H.R. & Seeley, T.D. 2007: Genetic diversity in honey bee colonies enhances productivity and fitness. – Science 317: 362-364.
Oldroyd, B.P. & Fewell, J.H. 2007: Genetic diversity promotes homeostasis in insect colonies. – Trends in Ecology & Evolution 22: 408-413.
Page, R.E., Robinson, G.E., Fondrk, M.K. & Nasr, M.E. 1995: Effects of worker genotypic diversity on honey bee colony development and behavior (Apis mellifera L.). – Behavioral Ecology and Sociobiology 36: 387-396.
Schwander, T., Lo, N., Beekman, M., Oldroyd, B.P. & Keller, L. 2010: Nature versus nurture in social insect caste differentiation. – Trends in Ecology & Evolution 25: 275-282.
Smith, C.R., Toth, A.L., Suarez, A.V. & Robinson, G.E. 2008: Genetic and genomic analyses of the division of labour in insect societies. – Nature Reviews Genetics 9: 735-748.
Smith, J.M. & Szathmary, E. 1997: The Major Transitions in Evolution. – Oxford University Press, Oxford, UK, 361 pp.
Trible, W., Olivos-Cisneros, L., McKenzie, S.K., Saragosti, J., Chang, N.-C., Matthews, B.J., Oxley, P.R. & Kronauer, D.J.C. 2017: orco Mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. – Cell 170: 727-735.
Ulrich, Y., Saragosti, J., Tokita, C.K., Tarnita, C.E. & Kronauer, D.J.C. 2018: Fitness benefits and emergent division of labour at the onset of group living. – Nature 560: 635-638.





Recent Comments