Ants and Fungi: a 66-million-year coevolutionary partnership
How did fungus-farming ants and their fungal cultivators coevolve? In a recent study, “The coevolution of fungus-ant agriculture,” published in Science, Schultz et al. answer that question by mapping the coevolutionary history of both partners using over 2000 genetic markers. Researchers found that ant agriculture emerged around 66 million years ago, likely spurred by the end-Cretaceous asteroid impact. They also traced the domestication of fungal cultivars 27 million years ago, coinciding with environmental changes in South America. The study highlighted a new perspective on positive selection in fungal genes shaping this remarkable mutualism, setting the stage for future comparative genomic studies on ant-fungal coevolution.
Here, Ted R. Schultz shares some of the study’s critical findings and insights.

An Interview compiled by Purbayan Ghosh and Salvatore Brunetti


MNB: Could you tell us a bit about yourself?
TS: I am a Research Entomologist at the Smithsonian Institution’s National Museum of Natural History in Washington, DC, U.S.A., where I have been employed since 1995. That year, at the age of 42, I earned my Ph.D. in Entomology from Cornell University, where I had been a graduate student since 1988. In that same year I finally completed a B.A. in Biology from U.C. Berkeley. During the twenty preceding years, I had been in and out of multiple undergraduate programs at various institutions (e.g., University of Chicago, Sonoma State University), studying subjects ranging from fine art to computer science. Along the way, I worked in dozens of diverse jobs, including railroad car inspector, taxi driver, bartender, dishwasher, dental technician, bicycle messenger, janitor, printer, upholsterer, magazine and newspaper production artist, copy-camera operator, proofreader, copy editor, managing editor, and freelance writer, among others.

At Cornell, under the guidance of the late, great ant taxonomist William L. Brown, Jr., I focused my research on fungus-farming ants and discovered the importance of field work. Around 1991, when my funding was running low, my wife, Rebecca, and I sold a significant portion of my comic book collection at a convention in New York City and I used the proceeds to fund a four-month fungus-farming ant expedition to Costa Rica, Nicaragua, and Brazil, including the Cerrado and Amazonian forests. That expedition was life-changing, both personally and professionally. Since then, I’ve conducted around thirty extended field trips to sites in Central and South America, particularly in Brazil, where I have built lasting friendships. My comparative research would not be possible without the specimens (ants and fungi) collected during these trips by my colleagues and myself.

MNB: Could you briefly outline your research on “ The coevolution of fungus-ant agriculture ” published in Science in layman’s terms?
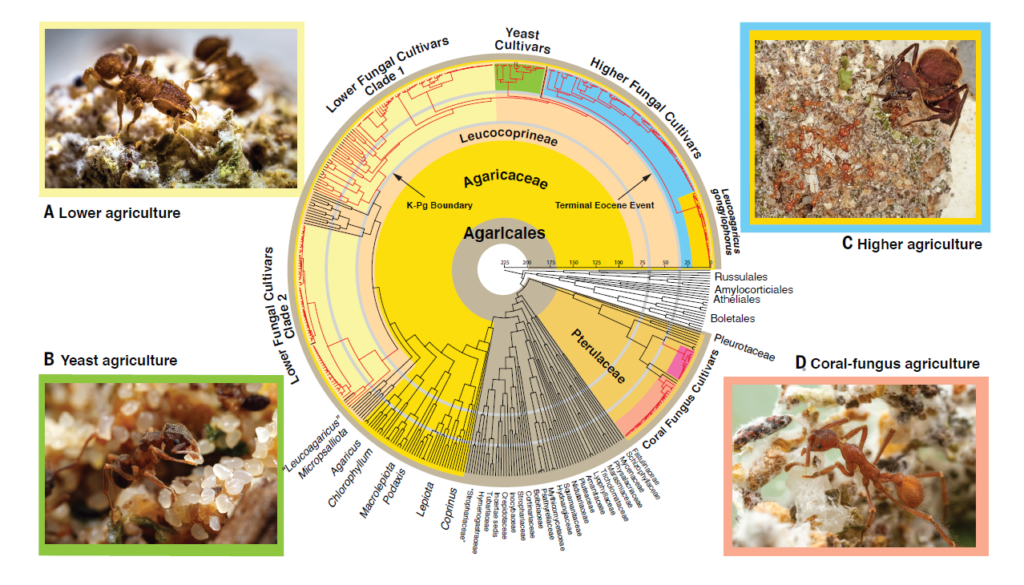
TS: The paper was a collaborative effort involving 23 coauthors, including bioinformaticians, mycologists, and myrmecologists. Together, we constructed two time-dated evolutionary trees (“chronograms”), one for ants and one for fungi. The ant tree includes 276 ants, including 208 fungus-farming ants, and the fungal tree includes 475 fungi, including 288 ant-cultivated fungi. In 137 instances, ants and fungi were collected from the same nests. The fungal tree is based on data produced by genetic probes designed for this project by Vinson Doyle and Brant Faircloth, which target 2121 gene regions called Ultra-Conserved Elements (UCEs)- regions of the genome for which the cores remain relatively unchanged over evolutionary time. Using these probes, we created the first reliable evolutionary tree for the ant-cultivated fungi. Traditionally, ant-cultivated fungi have been divided into four major groups — lower, higher, yeast, and coral fungus cultivars — and our fungal chronogram reveals when each group diverged from non-ant cultivated ancestors and how the groups are related to one another. Our ant chronogram does the same thing for the groups of ants that cultivate the groups of fungi. This allowed us to examine historical correspondences across the fungal and ant chronograms.
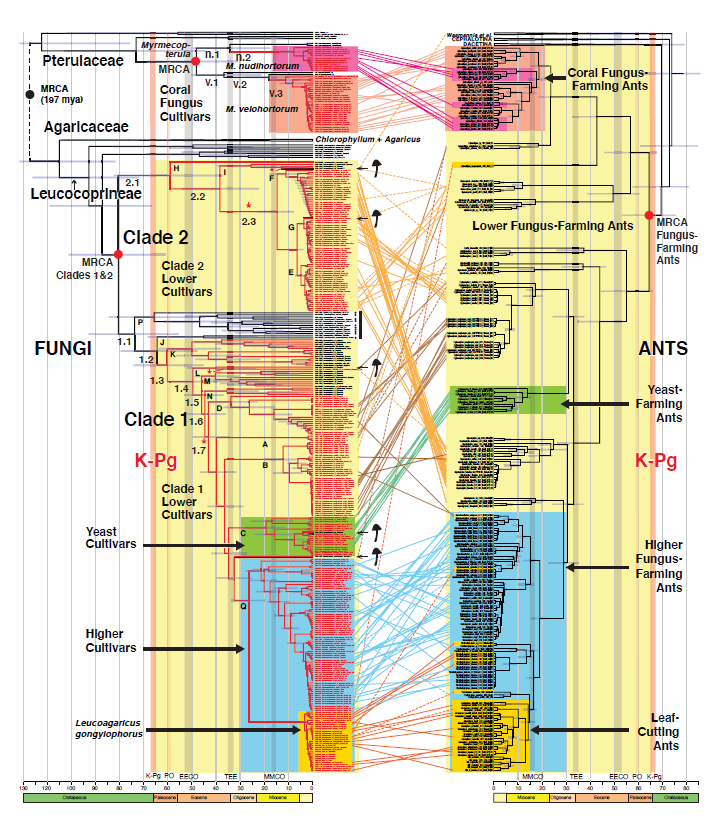
In brief, we found strong temporal concordance across the fungal and ant chronograms. Our data confirmed what we already knew: that cultivating fungi in the Leucocoprineae (i.e., species currently placed in the genera Leucocoprinus, Leucoagaricus, and Lepiota) was the ancestral form of fungus-ant agriculture, and that cultivating fungi in the Pterulaceae (i.e., coral-fungus species in the genus Myrmecopterula) arose much later. Our data also revealed that cultivation by ants arose not once, but twice in the Leucocoprineae and twice in Myrmecopterula.
Interestingly, we found that the origin of fungus-ant agriculture coincided with the end-of-Cretaceous extinction event, which wiped out non-avian dinosaurs and shut down photosynthesis for several months. The fossil record indicates that during this time period, trees and other plants died off and fungi briefly proliferated (Carvalho et al. 2021; Vajda & McLoughlin 2004). What was catastrophic for some species turned out to be a golden opportunity for leaf-litter-digesting fungi and leaf-litter-dwelling ants, especially because, as we hypothesize, they had already established a proto-agricultural relationship prior to the extinction event.

Our study also highlighted the emergence of higher fungi around 27 million years ago, along with the higher fungus-farming ants that cultivate them. We consider the higher fungi to be “domesticated” under any definition of the word because, unlike the lower cultivars and the yeast cultivars, they are never found living in the leaf litter independent of their ant farmers. Also, the higher fungi are multinucleate or polyploid (i.e., they have more than the two nuclei per cell typical of most fungi) and they consistently produce food bodies called gongylidia, which the ants preferentially harvest and consume. The origin of the higher cultivars and their farmers occurred shortly after the Terminal Eocene Event, a period of global cooling in which seasonally dry grasslands and woody savannahs expanded in South America, fragmenting formerly continuous wet Neotropical forests. We had previously hypothesized that, as the ants adapted to the drier habitats and brought their fungal cultivars with them, the cultivars were isolated from their wet-forest-dwelling, free-living, extended gene pools (Branstetter et al. 2017). This would have facilitated the domestication process and rendered the cultivars dependent on the ant farmers.
MNB: What is the take-home message of your work?
TS: Major events in Earth’s history are correlated with major transitions in the coevolution of fungus-ant agriculture. Additionally, the evolution of ant agriculture shares parallels with the evolution of human agriculture, particularly with regard to proto-agriculture and domestication.

MNB: What was your motivation for this study?
TS: I have always been fascinated by the complex behaviors of ants. Beyond their social behaviors, ants display a wide range of complex behaviors- herding and “milking” homopterans, waging wars against each other, kidnapping pupae from other species to become their own workers, protecting plant mutualists from herbivores, and more. Of all these complex behaviors, I have always found agriculture to be the most captivating: How did agriculture evolve in ants? As a graduate student I decided that I would largely focus on this simple question and that question continues to motivate much of my research today.

MNB: What was the biggest obstacle you had to overcome in this project?
TS: Early in my graduate studies, I realized that to understand how agriculture evolved in ants, I need to understand the evolution of the ant-cultivated fungi. I started to collaborate with mycologists and over the years we’ve attempted, with limited success, to reconstruct the evolution of the ant-cultivated fungi. Unfortunately, however, we’ve been limited by the inadequacy of the genetic markers available. Finally, after all these years, we overcame this limitation using Doyle’s and Faircloth’s UCE phylogenomic probes. Even with the probes, however, this project wouldn’t have been possible without the extensive collection of both ant-cultivated and non-ant-cultivated fungal samples by many people over many years.

MNB: Do you have any tips for others who are interested in doing related research?
TS: Field work is important. Go to where the organisms live and observe them on their own terms. You will almost certainly encounter surprises.


MNB: Where do you see the future for this particular field of ant research?
TS: Our study shows that, on the fungal tree, the highest rate of positive selection in UCE protein-coding loci of any branch we measured occurred on the branch leading to the higher fungal cultivars. Significantly high rates characterize the origins of the other fungal groups as well. The next logical step is to obtain whole genomes from critical fungi and ants sampled from both sides of these evolutionary origins, ancestral and derived. Fortunately, our chronograms identify the most relevant fungal and ant species to sample to understand what genes were involved in the origins of proto-agriculture, lower agriculture, yeast agriculture, higher agriculture, and coral-fungus agriculture. Since we do not have all of these fungal species in axenic culture, further field work will be needed to recollect them and get them into culture.






A very good and interesting article!
Maybe this link will help:
https://www.ameisenhaltung.de/attachment/19490-the-coevolution-of-fungus-ant-agriculture-pdf/